Enerji yoğunluğu
Enerji yoğunluğu birim hacim başına belirli bir sistemde saklanan enerji miktarıdır. Genelde, yalnızca kullanılabilir ya da elde edilebilir enerji miktarı göz önüne alınır. Bir başka deyişle, örneğin durağan kütlenin enerjisi ihmal edilir.[1]
Yakıtlar için, birim hacim başına enerji kullanışlı bir parametredir. Örneğin, hidrojen yakıtı ile benzin kıyaslanırsa, hidrojen daha yüksek özgül enerjiye sahip iken, daha düşük enerji yoğunluğuna sahiptir (sıvı halde iken dahi).
Birim hacim başına enerji birimi, basınç ile aynı fiziksel birime sahiptir ve çoğu durumda bununla eşanlamlı olarak da kullanılabilir: örneğin, bir manyetik alanının enerji yoğunluğu basınç olarak ifade edilir.
Yakıt ve enerji depolamada enerji yoğunluğu
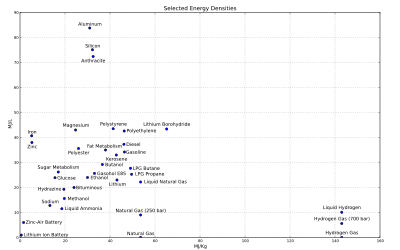
Enerji depolama uygulamalarinda enerji yogunlugu depo agirligi ile depo hacmini birbiri ile baglantilandirir, or. yakit tankinda. Yuksek enerji yogunluklu yakit ile, ayni hacim miktari için daha fazla enerji depo edilebilir ve tasinabilir. Bir yakitin birim kutle basina dusen enerji yogunlugu, yakitin ozgul enerjisi olarak tanimlanir.
Madde, kutlesi ile en buyuk enerji kaynagidir. Bu enerji, E=mc^2 formulu ile gosterilir (m=ρV; ρ maddenin yogunlugu; V kutlenin hacmi ve c isik hizidir.) Bu enerji ancak nukleer fizyon veya fuzyon ile serbest kalabilir. Nukleer tepkimeler ise kimyasal tepkimelere (or. yanma) benzetilemez.
Gercek enerji yogunluklari
Bu tablo This table gives the energy density of a complete system, including all required external components, such as oxidisers or heat sources. 1 MJ ≈ 0.28 kWh ≈ 0.37 HPh.
| Depolama turu | Ozgul energy (MJ/kg) | Energy density (MJ/L) | Specific energy density (Pm·kg/s4) | Peak recovery efficiency % | Practical recovery efficiency % |
|---|---|---|---|---|---|
| Indeterminate matter and antimatter | ≈8.9876e10 | 5e25[2] | 5e36 | ||
| Deuterium-tritium fusion | 576,000,000 | ||||
| Uranium-235 used in nuclear weapons | 88,250,000 | ||||
| Natural uranium (99.3% U-238, 0.7% U-235) in fast breeder reactor | 86,000,000[3] | ||||
| Reactor-grade uranium (3.5% U-235) in light water reactor | 3,456,000 | 30% | |||
| Pu-238 α-decay | 2,200,000 | ||||
| Hf-178m2 isomer | 1,326,000 | 17,649,060 | 2.340e13 | ||
| Natural uranium (0.7% U235) in light water reactor | 443,000 | 30% | |||
| Ta-180m isomer | 41,340 | 689,964 | 2.852e10 | ||
| Zip fuel | 70 | ||||
| Specific orbital energy of low Earth orbit (approximate) | 33 | ||||
| Beryllium and oxygen | 23.9[4] | ||||
| Lithium and fluorine | 23.75 | ||||
| Octaazacubane (potential explosive) | 22.9[5] | ||||
| Dinitroacetylene explosive - computed | 9.8 | ||||
| Octanitrocubane explosive | 8.5[6] | 16.9[7] | 144 | ||
| Tetranitrotetrahedrane explosive - computed | 8.3 | ||||
| Heptanitrocubane explosive - computed | 8.2 | ||||
| Sodium (reacted with chlorine) | 7.0349 | ||||
| Hexanitrobenzene explosive | 7[8] | ||||
| Tetranitrocubane explosive - computed | 6.95 | ||||
| Ammonal (Aluminium and NH4NO3 oxidizer) | 6.9 | 12.7 | 88 | ||
| Tetranitromethane and hydrazine bipropellant - computed | 6.6 | ||||
| Nitroglycerin | 6.38[9] | 10.2[10] | 65.1 | ||
| ANFO-ANNM | 6.26 | ||||
| Octogen (HMX) | 5.7[9] | 10.8[11] | 62 | ||
| TNT [12] | 4.610 | 6.92 | 31.9 | ||
| Copper thermite (aluminium and CuO as oxidizer) | 4.13 | 20.9 | 86.3 | ||
| Thermite (powdered aluminium and Fe2O3 as oxidizer) | 4.00 | 18.4 | 73.6 | ||
| Hydrogen peroxide decomposition (as monopropellant) | 2.7 | 3.8 | 10 | ||
| Battery, lithium ion nanowire | 2.54 (claimed) | 95%[13] | |||
| Battery, lithium thionyl chloride (LiSOCl2)[14] | 2.5 | ||||
| Water 220.64 bar, 373.8 °C | 1.968 | 0.708 | 1.393 | ||
| Kinetic energy penetrator | 1.9 | 30 | 57 | ||
| Battery, hydrogen closed-cycle fuel cell[15] Şablon:Smn | 1.62 | ||||
| Hydrazine (toxic) decomposition (as monopropellant) | 1.6 | 1.6 | 2.7 | ||
| Ammonium nitrate decomposition (as monopropellant) | 1.4 | 2.5 | 3.5 | ||
| Thermal energy capacity of molten salt | 1 | 98%[16] | |||
| Molecular spring approximate | 1 | ||||
| Battery, sodium sulfur | 0.72[17] | 1.23 | 0.89 | 85%[18] | |
| Battery, lithium-manganese[19][20] | 0.83-1.01 | 1.98-2.09 | 1.64-2.11 | ||
| Battery, lithium ion[21][22] | 0.46-0.72 | 0.83-3.6[23] | 0.38-2.6 | 95%[24] | |
| Battery, lithium sulfur[25] | 1.80[26] | 1.80 | 3.2 | ||
| Battery, sodium nickel chloride, High Temperature | 0.56 | ||||
| Battery, silver oxide[19] | 0.47 | 1.8 | 0.85 | ||
| Flywheel | 0.36-0.5[27][28] | ||||
| 5.56 × 45 mm NATO bullet | 0.4 | 3.2 | 1.3 | ||
| Battery, nickel metal hydride (NiMH), low power design as used in consumer batteries[29] | 0.4 | 1.55 | 0.62 | ||
| Battery, zinc-manganese (alkaline), long life design[19][21] | 0.4-0.59 | 1.15-1.43 | 0.46-0.84 | ||
| Liquid nitrogen | 0.349 | ||||
| Water, enthalpy of fusion | 0.334 | 0.334 | 0.112 | ||
| Battery, zinc bromide flow (ZnBr)[30] | 0.27 | ||||
| Battery, nickel metal hydride (NiMH), High Power design as used in cars[31] | 0.250 | 0.493 | 0.123 | ||
| Battery, nickel cadmium (NiCd)[21] | 0.14 | 1.08 | 0.15 | 80%[24] | |
| Battery, zinc-carbon[21] | 0.13 | 0.331 | 0.043 | ||
| Battery, lead acid[21] | 0.14 | 0.36 | 0.050 | ||
| Battery, vanadium redox | 0.09 | 0.1188 | 0.011 | 70-75% | |
| Battery, vanadium bromide redox | 0.18 | 0.252 | 0.045 | 80%–90%[32] | |
| Capacitor, ultracapacitor | 0.019597 (max)[33] | 0.025568(max)[33] | 0.00100 | ||
| Capacitor, supercapacitor | 0.01 | 80%–98.5%[34] | 39%–70%[34] | ||
| Rubber strip motor | 0.01[35] | ||||
| Superconducting magnetic energy storage | 0.008[36] | >95% | |||
| Capacitor | 0.002[37] | ||||
| Neodymium magnet | 0.003[38] | ||||
| Ferrite magnet | 0.0003[38] | ||||
| Spring power (clock spring), torsion spring | 0.0003[39] | 0.0006 | 0.00000018 | ||
| Storage type | Energy density by mass (MJ/kg) | Energy density by volume (MJ/L) | Specific energy density (Pm·kg/s4) | Peak recovery efficiency % | Practical recovery efficiency % |
Kaynakça
- ↑ http://physics.nist.gov/Pubs/SP811/sec04.html
- ↑ Assumes density of a neutron star
- ↑ "Facts from Cohen". Formal.stanford.edu. 2007-01-26. 18 Kasım 2015 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20151118212257/http://www-formal.stanford.edu/jmc/progress/cohen.html. Erişim tarihi: 2013-06-01.
- ↑ "The Heat of Formation of Beryllium Oxide1 - Journal of the American Chemical Society (ACS Publications)". Pubs.acs.org. 2002-05-01. http://pubs.acs.org/doi/abs/10.1021/ja01109a018. Erişim tarihi: 2010-05-07.
- ↑ "Besides N2, What Is the Most Stable Molecule Composed Only of Nitrogen Atoms?† - Inorganic Chemistry (ACS Publications)". Pubs.acs.org. 1996-05-28. http://pubs.acs.org/doi/abs/10.1021/ic9606237. Erişim tarihi: 2010-05-07.
- ↑ http://www3.interscience.wiley.com/journal/122324589/abstract
- ↑ "Octanitrocubane - Wikipedia, the free encyclopedia". En.wikipedia.org. 8 Ekim 2015 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20151008150909/https://en.wikipedia.org/wiki/Octanitrocubane. Erişim tarihi: 2010-05-07.
- ↑ http://www3.interscience.wiley.com/journal/109618256/abstract
- 1 2 "Chemical Explosives". Fas.org. 2008-05-30. 27 Mayıs 2015 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20150527001638/http://fas.org:80/man/dod-101/navy/docs/es310/chemstry/chemstry.htm. Erişim tarihi: 2010-05-07.
- ↑ Česky. "Nitroglycerin - Wikipedia, the free encyclopedia". En.wikipedia.org. 11 Aralık 2015 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20151211013616/https://en.wikipedia.org/wiki/Nitroglycerin. Erişim tarihi: 2010-05-07.
- ↑ Česky (2010-05-01). "HMX - Wikipedia, the free encyclopedia". En.wikipedia.org. 7 Kasım 2015 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20151107115903/https://en.wikipedia.org/wiki/HMX. Erişim tarihi: 2010-05-07.
- ↑ Kinney, G.F.; K.J. Graham (1985). Explosive shocks in air. Springer-Verlag. ISBN 3-540-15147-8.
- ↑ "Nanowire battery can hold 10 times the charge of existing lithium-ion battery". News-service.stanford.edu. 2007-12-18. 7 Ocak 2010 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20100107182920/http://news-service.stanford.edu/news/2008/january9/nanowire-010908.html. Erişim tarihi: 2010-05-07.
- ↑ "Lithium Thionyl Chloride Batteries". Nexergy. 24 Şubat 2011 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20110224203027/http://www.nexergy.com:80/lithium-thionyl-chloride.htm. Erişim tarihi: 2010-05-07.
- ↑ "The Unitized Regenerative Fuel Cell". Llnl.gov. 1994-12-01. 24 Şubat 2013 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20130224134547/https://www.llnl.gov/str/Mitlit.html. Erişim tarihi: 2010-05-07.
- ↑ "Technology". SolarReserve. 1 Şubat 2012 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20120201161512/http://www.solar-reserve.com:80/technology.html. Erişim tarihi: 2010-05-07.
- ↑ "New battery could change world, one house at a time". Heraldextra.com. 2009-04-04. 17 Ekim 2015 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20151017163418/http://www.heraldextra.com/news/article_b0372fd8-3f3c-11de-ac77-001cc4c002e0.html. Erişim tarihi: 2010-05-07.
- ↑ "Energy Citations Database (ECD) - - Document #5960185". Osti.gov. 14 Şubat 2012 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20120214102044/http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=5960185. Erişim tarihi: 2010-05-07.
- 1 2 3 "ProCell Lithium battery chemistry". Duracell. 7 Eylül 2011 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20110907092418/http://www1.duracell.com:80/procell/chemistries/lithium.asp. Erişim tarihi: 2009-04-21.
- ↑ "Properties of non-rechargeable lithium batteries". corrosion-doctors.org. 29 Nisan 2014 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20140429072647/http://www.corrosion-doctors.org:80/PrimBatt/table2.htm. Erişim tarihi: 2009-04-21.
- 1 2 3 4 5 "Battery energy storage in various battery types". AllAboutBatteries.com. 21 Eylül 2015 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20150921003751/http://www.allaboutbatteries.com/Battery-Energy.html. Erişim tarihi: 2009-04-21.
- ↑ A typically available lithium ion cell with an Energy Density of 201 wh/kg
- ↑ "Lithium Batteries". 8 Ağustos 2011 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20110808112807/http://www.globalspec.com/Specifications/Electrical_Electronic_Components/Batteries/Lithium_Batteries. Erişim tarihi: 2010-07-02.
- 1 2 Justin Lemire-Elmore (2004-04-13). "The Energy Cost of Electric and Human-Powered Bicycles". ss. 7. 13 Eylül 2012 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20120913095738/http://www.ebikes.ca/sustainability/Ebike_Energy.pdf. Erişim tarihi: 2009-02-26. "Table 3: Input and Output Energy from Batteries"
- ↑ "Lithium Sulfur Rechargeable Battery Data Sheet". Sion Power, Inc.. 2005-09-28. 28 Ağustos 2008 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20080828105501/http://www.sionpower.com/pdf/sion_product_spec.pdf.
- ↑ Kolosnitsyn, V.S.; E.V. Karaseva (2008). "Lithium-sulfur batteries: Problems and solutions". Russian Journal of Electrochemistry (Maik Nauka/Interperiodica/Springer) 44: 506–509. DOI:10.1134/s1023193508050029.
- ↑ Storage Technology Report, ST6 Flywheel
- ↑ "Next-gen Of Flywheel Energy Storage". Product Design & Development. 4 Eylül 2012 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20120904124210/http://www.pddnet.com:80/article-next-gen-of-flywheel-energy-storage/. Erişim tarihi: 2009-05-21.
- ↑ Advanced Materials for Next Generation NiMH Batteries, Ovonic, 2008
- ↑ "ZBB Energy Corp". 2007-10-15 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20071015134212/http://zbbenergy.com/technology.htm. "75 to 85 watt-hours per kilogram"
- ↑ High Energy Metal Hydride Battery
- ↑ "Microsoft Word - V-FUEL COMPANY AND TECHNOLOGY SHEET 2008.doc" (PDF). 22 Ağustos 2011 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20110822100235/http://www.vfuel.com.au/infosheet.pdf. Erişim tarihi: 2010-05-07.
- 1 2 "Nesscap Data Sheet". Nesscap.com. http://www.nesscap.com/data_nesscap/spec_sheets/Spec%2009.pdf. Erişim tarihi: 2011-02-24.
- 1 2 http://www2.fs.cvut.cz/web/fileadmin/documents/12241-BOZEK/publikace/2004/Sup-Cap-Energy-Storage.pdf
- ↑ various modelling sources quote ‘4,000 ft-lb/lb'
- ↑
- ↑ http://www.doc.ic.ac.uk/~mpj01/ise2grp/energystorage_report/node9.html
- 1 2 http://www.askmar.com/Magnets/Promising%20Magnet%20Applications.pdf
- ↑ "Garage Door Springs". Garagedoor.org. 13 Ekim 2008 tarihinde kaynağından arşivlendi. http://web.archive.org/web/20081013121817/http://garagedoor.org/residential/torsion-springs.php. Erişim tarihi: 2010-05-07.